Prosultiamine
Appearance
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.397 |
| Chemical and physical data | |
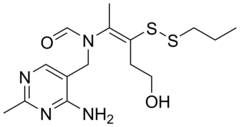
| Formula | C15H24N4O2S2 |
| Molar mass | 356.50 g·mol−1 |
| 3D model (JSmol) | |
| |
Prosultiamine (INN; also known as thiamine propyl disulfide or TPD; brand name Jubedel,) is a disulfide thiamine derivative discovered in garlic in Japan in the 1950s, and is similar to allithiamine. It was developed as a treatment for vitamin B1 deficiency.[1][2] [3] It has improved lipid solubility relative to thiamine and is not rate-limited by dependency on intestinal transporters for absorption, hence the reasoning for its development.[4][5]
Research
[edit]It has been studied as a potential treatment for infection with human T-lymphotropic virus (HTLV), since it has been shown to reduce viral load and symptoms.[6]
See also
[edit]References
[edit]- ^ Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. ISBN 3-88763-075-0.
- ^ Triggle DJ (1997). Dictionary of pharmacological agents. London: Chapman & Hall. ISBN 0-412-46630-9.
- ^ Fujiwara M, Watanabe H, Matsui K (1954). ""allithiamine" A Newly Found Derivative of Vitamin B1". The Journal of Biochemistry. 41: 29–39. doi:10.1093/oxfordjournals.jbchem.a126421.
- ^ Thomson AD, Frank O, Baker H, Leevy CM (April 1971). "Thiamine propyl disulfide: absorption and utilization". Annals of Internal Medicine. 74 (4): 529–534. doi:10.7326/0003-4819-74-4-529. PMID 5551161.
- ^ Baker H, Frank O (August 1976). "Absorption, utilization and clinical effectiveness of allithiamines compared to water-soluble thiamines". Journal of Nutritional Science and Vitaminology. 22 SUPPL: 63–68. doi:10.3177/jnsv.22.supplement_63. PMID 978282.
- ^ "Nervous System Disease: A New Outlet for an Old Drug?". Science Daily. 15 August 2013.