n-Butanol
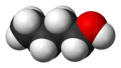
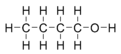
n-Butanol (n-butil alkohol, normalni butanol) je primarni alkohol sa četvorougljeničnom strukturom i molekulskom formulom C4H9OH. Njegovi izomeri su izobutanol, 2-butanol, i tert-butanol.
| n-Butanol | |||
|---|---|---|---|
 | |||
| |||
| Naziv po klasifikaciji | Butan-1-ol[1] | ||
| Drugi nazivi | Butalkohol Butanol | ||
| Identifikacija | |||
| CAS registarski broj | 71-36-3 | ||
| PubChem[2][3] | 263 | ||
| ChemSpider[4] | 258 | ||
| UNII | 8PJ61P6TS3 | ||
| EINECS broj | |||
| UN broj | 1120 | ||
| DrugBank | DB02145 | ||
| KEGG[5] | |||
| MeSH | |||
| ChEBI | 28885 | ||
| ChEMBL[6] | CHEMBL14245 | ||
| RTECS registarski broj toksičnosti | EO1400000 | ||
| Bajlštajn | 969148 | ||
| Gmelin Referenca | 25753 | ||
| 3DMet | B00907 | ||
| Jmol-3D slike | Slika 1 | ||
| |||
| |||
| Svojstva | |||
| Molekulska formula | C4H10O | ||
| Molarna masa | 74.12 g mol−1 | ||
| Agregatno stanje | Bezbojna tečnost | ||
| Gustina | 0,81 g cm-3 | ||
| Tačka topljenja |
−90 °C, 183 K, -130 °F | ||
| Tačka ključanja |
118 °C, 391 K, 244 °F | ||
| Rastvorljivost u vodi | 73 g L-1 na 25 °C | ||
| log P | 0,839 | ||
| Indeks prelamanja (nD) | 1,399 (20 °C) | ||
| Viskoznost | 3 cP | ||
| Dipolni moment | 1,66 D | ||
| Termohemija | |||
| Standardna entalpija stvaranja jedinjenja ΔfH |
−328(4) kJ mol-1 | ||
| Std entalpija sagorevanja ΔcH |
−2670(20) kJ mol-1 | ||
| Standardna molarna entropija S |
225,7 J K−1 mol−1 | ||
| Opasnost | |||
| Podaci o bezbednosti prilikom rukovanja (MSDS) | ICSC 0111 | ||
| EU-klasifikacija | |||
| EU-indeks | 603-004-00-6 | ||
| NFPA 704 | |||
| R-oznake | R10, R22, R37/38, R41, R67 | ||
| S-oznake | S2, S7/9, S13, S26, S37/39, S46 | ||
| Tačka paljenja | 35 °C | ||
| Tačka spontanog paljenja | 343 °C | ||
| Eksplozivni limiti | 1,4–11,2% | ||
| Srodna jedinjenja | |||
| Srodna jedinjenja | Butanetiol | ||
|
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje (25 °C, 100 kPa) materijala | |||
| Infobox references | |||
n-Butanol se prirodno javlja kao manje zastupljeni proizvod fermentacije šećera i drugih ugljenih hidrata,[7] i prisutan je u mnogim vrstama hrane i pića.[8][9] On se takođe koristi kao veštačka aroma,[10] u puteru, kremovima, rumu, viskiju, sladoledu, bombonama, i pecivu.[11] It is also used in a wide range of consumer products.[8]
Reference
uredi- ↑ „1-Butanol - Compound Summary”. The PubChem Project. USA: National Center of Biotechnology Information.
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519.
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846.
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594.
- ↑ Hazelwood, Lucie A.; Daran, Jean-Marc; van Maris, Antonius J. A.; Pronk, Jack T.; Dickinson, J. Richard (2008), „The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism”, Appl. Environ. Microbiol. 74 (8): 2259–66, DOI:10.1128/AEM.02625-07, PMC 2293160, PMID 18281432.
- ↑ 8,0 8,1 Butanols: four isomers, Environmental Health Criteria monograph No. 65, Geneva: World Health Organization, 1987, ISBN 92-4-154265-9.
- ↑ n-Butanol, SIDS Initial Assessment Report, Geneva: United Nations Environment Programme, April 2005.
- ↑ 21 C.F.R. § 172.515; 42 FR 14491, Mar. 15, 1977, as amended.
- ↑ Hall, R. L.; Oser, B. L. (1965), „Recent progress in the consideration of flavouring ingredients under the food additives amendement. III. Gras substances”, Food Technol.: 151, cited in Butanols: four isomers, Environmental Health Criteria monograph No. 65, Geneva: World Health Organization, 1987, ISBN 92-4-154265-9.
Spoljašnje veze
uredi- Internacionalna karta hemijske bezbednosti 0111
- Nacionalni institut za okupacionu bezbednost i zdravlje (NIOSH) Džepni vodič hemijskih hazarda 0076
- SIDS Inicijalni izveštaj o proceni for n-Butanol Organizacije za ekonomsku saradnju i razvoj (OECD)
- IPCS Ekološki zdravstveni kriterijum 65: Butanols: four isomers

